A female in her thirties presents to an emergency room in Miami, Florida with fever, headache, and flank pain.
This case will guide you through a clinical encounter to understand the patient's health challenges – and how to respond.
LEARNING OBJECTIVES
Understand how climate change can affect risks for vector-borne diseases
Identify sentinel cases of vector-borne disease related to climate change
Understand the role of the clinician in the diagnosis, management, and prevention of vector-borne diseases
CLINICAL COMPETENCIES
Integrate history of vector exposure into patient history
Develop a differential diagnosis for vector-borne disease
Understand and prioritize diagnostic testing for vector-borne disease
Identify populations at increased vulnerability to vector-borne disease
Access and integrate surveillance data / local outbreak and entomology data to improve diagnostic accuracy
Detect sentinel cases of vector borne disease
Integrate weather data/extreme events and local environmental factors to inform the differential diagnosis
Access resources to guide clinical management of climate sensitive infections
Educate patients, communities, and policy makers about prevention and the link between vector-borne disease and climate change
A Continuing Medical Education activity presented by the Stanford Center for Innovation in Global Health and the University of Washington. View CE information and claim credit HERE.
Accreditation
In support of improving patient care, Stanford Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Credit Designation
American Medical Association (AMA)
Stanford Medicine designates this Enduring Material for a maximum of 1.5 AMA PRA Category 1 Credits™.
Physicians should claim only the credit commensurate with the extent of their participation in the activity.
American Nurses Credentialing Center (ANCC)
Stanford Medicine designates this Enduring Material for a maximum of 1.5 ANCC contact hours.
American Academy of Physician Associates (AAPA)
STAnford Medicine has been authorized by the American Academy of PAs (AAPA) to award AAPA Category 1 CME credit for activities planned in accordance with AAPA CME Criteria.
This enduring activity is designated for 1.5 AAPA Category 1 CME credits. Approval is valid until December 14th, 2026. PAs should only claim credit commensurate with the extent of their participation.
Clinical Approach
Consider the following questions as you work through this case:
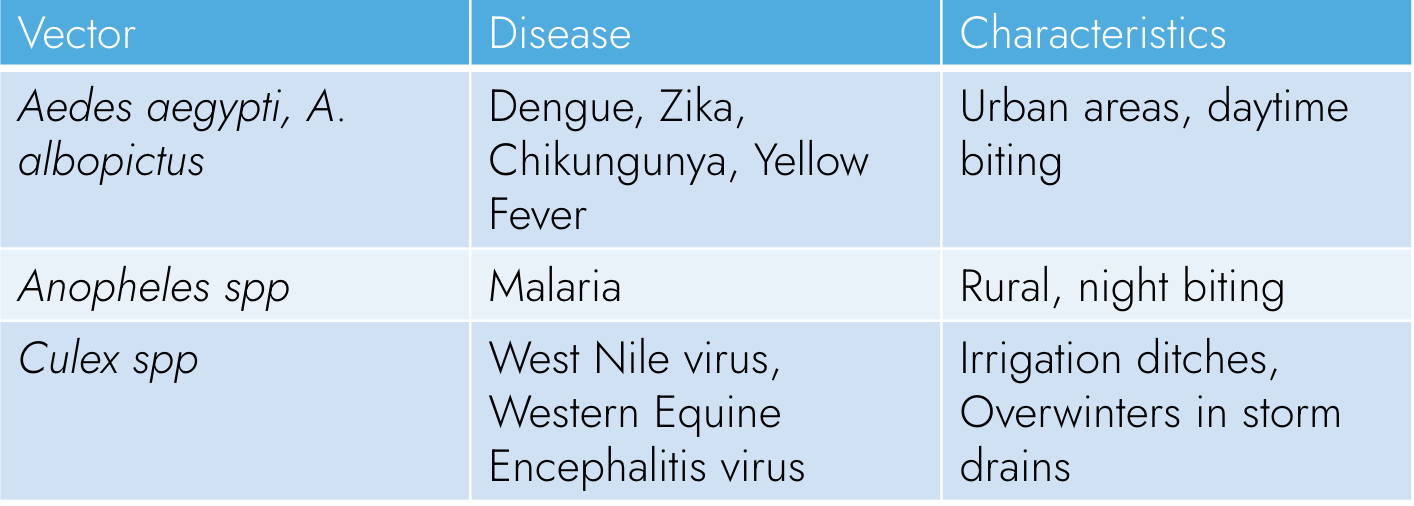
Vector-borne diseases result from infections transmitted to humans and other animals by vectors including mosquitoes, ticks, and fleas.
Key steps in diagnosis of vector borne disease:
History, inquire about bites, precautions taken (e.g., use of insect repellent, bed nets), and sentinel cases nearby, human or animal. Testing for the right diseases with the right tools can allow for earlier treatment once the final diagnosis is certain.
Understand the prevalent vectors in your area and know how to use risk maps and weather data to evaluate the risk of vector-borne diseases where you practice.
Access to data on vector risk can improve diagnosis.
History
Taking a clinical history of your patient yields the following information:
Chief complaint
History of present illness
Past medical & surgical history
Medications
Allergies
Family history
Social & enviromental history ("Social-E")
Review of systems
Discussion
What else would you want to know? What conditions are you already considering for your differential diagnosis?
What parts of the physical exam would you pay closer attention to?
Key Questions for Patient
Asking your patient the following questions gives you this information:
Are you immunocompromised in any way?
When was your last menstrual period?
Did you come into contact with anyone with TB or other respiratory diseases? Did you have sex while you were abroad?
Did you come into contact with untreated freshwater? Unpasteurized dairy or raw food? Soil?
Were you bitten by an insect or other animal recently, either when traveling or at home?

Physical Exam
When you examine your patient, you learn the following:
General
Head Eyes Ears Nose Throat (HEENT)
Neck
Chest & lungs
Cardiovascular
Abdominal
Genitourinary
Skin
Musculoskeletal
Neurological
Discussion
What is your differential diagnosis?
Based on the clinical context, is there any additional testing you would pursue?
Diagnostics
Information that will be helpful in diagnosing your patient:
Labs:
Complete blood count shows low white blood cell count (2,600 cells/u3) otherwise normal
Metabolic panel, serum lactate, coagulation studies, and urinalysis normal
Malaria parasite smear negative
Influenza RT-PCR negative
Chest x-ray normal
Ultrasound: Cholelithiasis, echogenic liver, normal common bile duct diameter
Abdominal CT scan: Gallbladder distension and wall thickening with pericholecystic fluid
Repeat labs hospital day 1:
New thrombocytopenia (platelets 62,000)
White blood cell count 15,000 cells/uL
Aspartate aminotransferase [AST] 265 units/L (normal: 15–46)
Alanine aminotransferase [ALT] = 100 units/L (normal: 9–52)
Alkaline phosphatase [ALP] = 164 units/L (normal: 38–126)
Key Points: Diagnostics
CONSIDER THE FOLLOWING INFORMATION:
Tourniquet test: Inflate BP cuff between systolic and diastolic for 5 min, observe 10 or more petechiae per 2.5 cm (1 inch) square
CBC with differential:
Leukopenia – viral infection (ie. HIV, Dengue), Typhoid
Thrombocytopenia – malaria, Dengue, HIV, typhoid, sepsis/DIC
CMP: Pay attention to elevated LFTs
Blood cultures: Two sets prior to antibiotics
Thick and thin blood smears; rapid diagnostic testing for Malaria: Three thick films/RDTs over 72 hours to exclude malaria with confidence
UA with urine culture: Proteinuria and hematuria in leptospirosis
Nucleic acid amplification testing (NAAT), serology, antigen test:
Acute and convalescent serum (SLOW turnaround, limited specificity)
NS1 antigen test for dengue – first seven days of illness
PCR – first 5 days of illness
Whole genome sequencing – can determine source

Differential Diagnosis
For altered mental status, fever, and respiratory distress, here are some etiologies you should consider:
Fever suggests infectious cause, but consider non-infectious intra-abdominal process as well.
Broad differential, including:
Urinary tract infection, pyelonephritis
Appendicitis, other intra-abdominal processes such as cholecystitis
Gastroenteritis
Viral illnesses (myalgia, arthralgia suggests viral)
Consider pregnancy/ectopic/pelvic infection
For history: Think vulnerability, geography & timing
Vulnerability: Immunocompromised? Pregnant?
Geography: For Florida and the Caribbean, influenza is common, and consider whether there are regional outbreaks of malaria, dengue, and Chikungunya. Recent data on infection incidence and vector abundance in your area are important!
Timing: Most pathogens (eg. arboviruses, hemorrhagic fever viruses, respiratory viruses, Rickettsiae) have an incubation period of < 21 days. Some, such as TB, malaria, protozoal, and HIV, have longer periods. Therefore, many travel-related infections would have presented earlier.
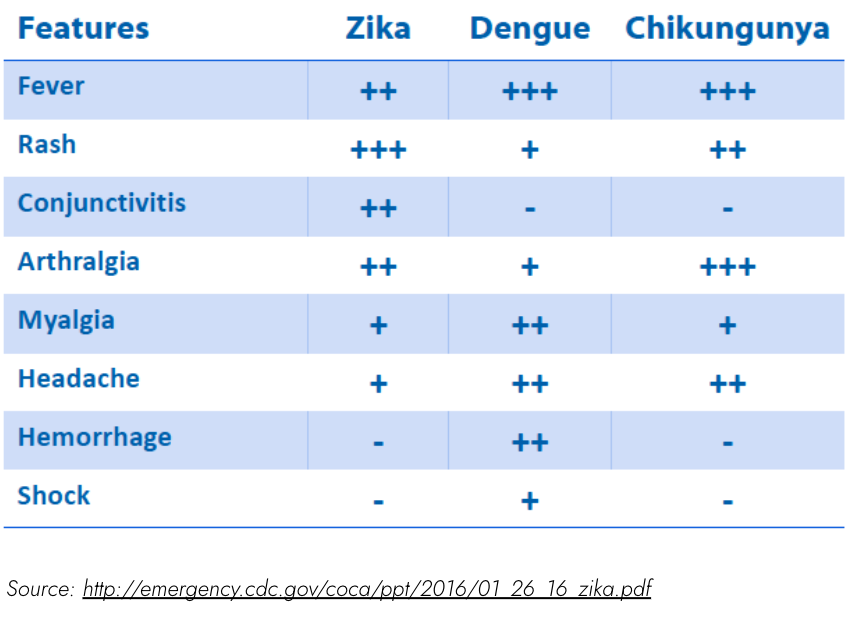
The myalgia and arthralgia and thrombocytopenia suggest arboviral illnesses – what are some to consider?
Some arboviral illnesses: Dengue, Zika, Chikungunya – some important similarities and differences.
Given the severe clinical picture (shock, thrombocytopenia), consider dengue!
*Islands of white within red maculopapular rash is pathognomonic for dengue
(Note: not all dengue patients have a rash!)

Final Diagnosis
the patient’s story continues – leaving you with a final diagnosis:
Dengue PCR positive, dengue antibody testing for IgG and IgM: dengue confirmed
In this case, travel to Honduras was too long ago for arbovirus infection acquired there, so this suggests local transmission.
Hemorrhagic presentation suggests dengue (but consider other causes of hemorrhagic fever, depending on geography: Lassa, Marburg, Ebola, Rift Valley Fever, Leptospirosis).
Shock, bleeding, organ failure indicates severe dengue (usually associated with reinfection).
Discussion
How do we treat dengue virus?
For viral diseases, including hemorrhagic fever, supportive care is critical mainstay.
Antivirals under development for arbovirus infections but are not in use.
For bacterial and parasitic diseases: Treat specific cause.
Management & Treatment
You can help your patient in the following ways:
Reducing host vulnerability / susceptibility (host factors):
Optimize host status / treat immunocompromised conditions
Reducing exposures / improving environments (environmental factors):
Be aware of high-risk areas and take extra precaution!
Avoid vector-prone habitat (marshy areas, high grass, etc.)
Personal protective equipment/clothing: Permethrin-impregnated clothing
Use of repellents:
DEET
Picaridin (known as KBR 3023 and icaridin outside the US)
Prevention
How can dengue virus infection be prevented?
*Customize use of the insect repellent that’s right for an individual
Beyond the Clinic
Your patient's story is part of a bigger picture:
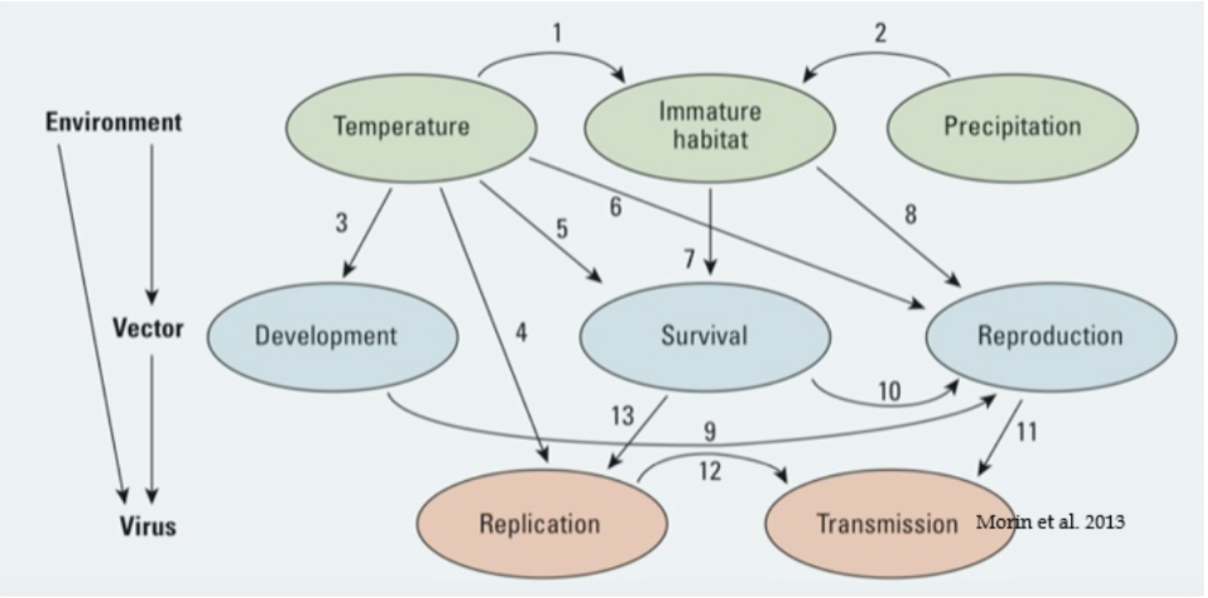
Climate change will also increase the burden of vector-borne disease. Due to changing environmental conditions and increasing average temperatures more hospitable to mosquitoes, dengue may be locally spread in eastern North America and southern Europe, and West Nile virus is predicted to be much more prevalent in all parts of the US by the end of this century. Warmer spring temperatures due to climate change may prompt an earlier start to mosquito activity and therefore lead to a longer virus amplification period for West Nile.
Aedes spp. mosquitoes (including Aedes aegypti and A. albopictus) are the vectors for a number of arboviruses, including dengue, Zika, Chikungunya, and yellow fever. Aedes mosquitoes are extending their range on several continents as a result of climate change. Zika virus and Chikungunya were previously unheard of in the United States but are now spreading locally as more species of mosquitoes, previously only found in the tropics, move northward. Urbanization, increased global trade, and increased global travel have further promoted the worldwide spread of vectors such as Aedes spp. Overall, disease-carrying mosquitoes will reach an additional 500 million people by 2050, including 55 million more Americans.
In addition to mosquitoes, other vectors, such as ticks, are prevalent in North America. Tick-borne diseases are becoming more prevalent as ticks expand their range, given the warming climate and milder winters. Ticks can spread infectious diseases like Lyme disease, babesiosis, anaplasmosis, and rickettsial disease, and they may also trigger alpha-gal allergy. Furthermore, Chagas disease, spread by the triatomine bug, and leishmaniasis, spread by sand flies, are also expanding their range in North America.
Given the potential devastating impacts of new vector-borne diseases in unprepared populations, it’s critical that “sentinel cases” be detected. Sentinel cases are events that indicate a changing disease risk – the “canary in the coal mine” or “tip of the iceberg” of an incoming onslaught of new vector-borne disease. For instance, birds are the natural reservoir for West Nile virus and are relatively unaffected by it; it has been shown that birds first carried West Nile from coast to coast in the United States in 1999. The first few cases can be in either humans or animals before ultimately spreading between humans, making communication between veterinarians and clinicians in human medicine even more important.
Source: CDC Vital Signs, May, 2018

Case Follow Up
What happens next:
The patient was hospitalized, given broad spectrum antibiotics and IV fluid boluses for suspicion of acute cholecystitis and sepsis.
On day 2 of entering the hospital, laparoscopic cholecystectomy performed; findings of gallbladder inflammation, diffuse bleeding from gallbladder fossa.
Day 3, the patient develops shock, abdominal distension, and lactic acidosis. Repeat surgery, complicated by Acute Respiratory Distress Syndrome (ARDS) and multi-organ failure. The patient receives empiric doxycycline for leptospira and rickettsia.
Day 4, the hospital sends blood studies for malaria, dengue, and Chikungunya.
After multiple transfusions and two additional surgeries for drainage, patient develops fungemia and cerebral edema and dies on day 29.
RNA sequencing of dengue virus in samples from patient showed dengue serotype 2: relationship to strain first seen in Cuba, now circulating in Miami: indicating that it was locally acquired in the United States.
After this “sentinel case,” several hundred additional imported cases were detected, including 19 locally acquired cases. This was evidence for spread of the vector risk.
Statewide clinician awareness education took place about avoiding the use of ibuprofen in suspected cases of dengue (to avoid bleeding), as well as dengue diagnosis and the need to report cases.
Hospital protocols were changed to automate molecular testing (PCR) for suspected dengue cases.
Public health conducted educational outreach in communities and airports about prevention of mosquito bites, recognition of febrile syndromes, and free testing for dengue and other arbovirus infection.
Discussion
What made the clinicians suspect vector-borne disease in the hospital after the first surgery on day 4?
How could clinicians have suspected and diagnosed dengue earlier?
Call to Action
Clinicians can take action to advance planetary health:
At the clinic:
Educate patients about vector-borne disease and prevention
Ensure clinicians have access to up to date vector risk maps and data
Ensure clinic has access to weather data that can affect vector risk
Report cases to health department or other relevant agencies
In your community:
Educate patients, communities, and policymakers about the role of climate change in vector-borne disease risk through meetings, social media, and op-eds
Communicate with veterinarians about sentinel cases of vector-borne disease and weather related outbreaks in humans and animals
At the societal level:
Raise awareness of the changing threat of vector-borne disease
Advocate for climate change mitigation measures at the local, state, and federal levels by voting and calling, emailing, and/or meeting with your representatives
Summary &
Key Learning Points
also available for download here:
Approach to diagnosis of vector-borne disease:
Key points in history and physical (eg. travel to endemic areas, history of any bites, history of precautions taken, history of sentinel cases nearby)
Understand biology of vectors
Use of risk maps and alerts – know what vector-borne diseases are occurring in your area!
Use of weather data – weather events can increase risk of vector-borne disease.
Sentinel cases – in humans and (when relevant) in animals – are an indicator of risk!
Appropriate diagnostics
Serology is less helpful in an acute care setting; instead molecular diagnostics (eg. Nucleic Acid Amplification Tests/NAATs) and rapid antigen tests are needed.
Management & treatment of vector-borne disease:
For viral diseases, supportive care is critical
Antivirals under development
Bacterial and parasitic diseases: Treat specific cause
Prevention:
Reducing host vulnerability / susceptibility (host factors)
Optimize host status / treat immunocompromised conditions
Reducing exposures / improving environments (environmental factors)
Be aware of high risk areas (maps, data, etc.) and educate patients in risk areas to take extra precaution!
Avoid vector-prone habitat (marshy areas, high grass, etc.)
Personal protective equipment/clothing: Permethrin-impregnated clothing
Use of repellents: DEET/ Picaridin, others

Additional Resources
Maps
HealthMap (worldwide map of infectious disease outbreaks)
ArboNET Disease Maps (CDC tool mapping number of human and animal cases of various infectious diseases by county in the US)
Vectorsurv (vector risk maps)
Datasets
ZOVER (database of zoonotic and vector-borne viruses from the Institute of Pathogen Biology of the Chinese Academy of Medical Sciences & Peking Union Medical College)
Vector-Borne Diseases in the United States, 2004-2018 (CDC dataset of vector-borne diseases by year, state, and specific disease)
Notifiable Infectious Disease Tables (updated weekly, from CDC)
Articles for further reading
Vector-borne diseases (WHO)
Surveillance Resources from the CDC based on vector and disease
Mapping Physiological Suitability Limits for Malaria in Africa Under Climate Change (Vector-Borne and Zoonotic Diseases)
Lyme disease: How climate change helped the illness invade America (Vox)
Estimated Effects of Projected Climate Change on the Basic Reproductive Number of the Lyme Disease Vector Ixodes scapularis (Environmental Health Perspectives)
Global expansion and redistribution of Aedes-borne virus transmission risk with climate change (PLOS Neglected Tropical Diseases)
Spread of The Tiger: Global Risk of Invasion by The Mosquito Aedes albopictus (Vector-Borne and Zoonotic Diseases)
Detecting the impact of temperature on transmission of Zika, dengue, and chikungunya using mechanistic models (PLOS Neglected Tropical Diseases)
Further Learning
Climate Resources for Health Education (CRHE) offers additional learning resources relevant to this case. Please explore the below links:

References
Ebi KL, Nealon J. Dengue in a changing climate. Environmental Research. 2016;151:115-123. doi:10.1016/j.envres.2016.07.026
Dostal T, Meisner J, Munayco C, García PJ, Cárcamo C, Pérez Lu JE, Morin C, Frisbie L, Rabinowitz PM. The effect of weather and climate on dengue outbreak risk in Peru, 2000-2018: A time-series analysis. PLoS Negl Trop Dis. 2022 Jun 30;16(6).
Kraemer MU, Sinka ME, Duda KA, Mylne AQ, Shearer FM, Barker CM, Moore CG, Carvalho RG, Coelho GE, Van Bortel W, Hendrickx G, Schaffner F, Elyazar IR, Teng HJ, Brady OJ, Messina JP, Pigott DM, Scott TW, Smith DL, Wint GR, Golding N, Hay SI. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. Elife. 2015 Jun 30;4:e08347.
Kraemer MUG, Reiner RC, Brady OJ, et al. Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nat Microbiol. 2019;4(5):854-863. doi:10.1038/s41564-019-0376-y
Reich S. What’s the Buzz? Birds, Horses, People Get West Nile Virus. University of Illinois College of Veterinary Medicine. Published July 24, 2018. https://vetmed.illinois.edu/pet-health-columns/birds-horses-people-west-nile-virus/
Ryan SJ, Carlson CJ, Mordecai EA, Johnson LR. Global expansion and redistribution of Aedes-borne virus transmission risk with climate change. Han BA, ed. PLoS Negl Trop Dis. 2019;13(3):e0007213. doi:10.1371/journal.pntd.0007213
Sharp TM, Morris S, Morrison A, de Lima Corvino D, Santiago GA, Shieh WJ, Rico E, Kopp E, Muñoz-Jordán JL, Marttos A, Paz-Bailey G, Abbo LM, Stanek D; 2019 Florida Dengue Investigation Team. Fatal Dengue Acquired in Florida. N Engl J Med. 2021 Jun 10;384(23):2257-2259.
Stephenson C, Coker E, Wisely S, Liang S, Dinglasan RR, Lednicky JA. Imported Dengue Case Numbers and Local Climatic Patterns Are Associated with Dengue Virus Transmission in Florida, USA. Insects. 2022 Feb 3;13(2):163.